Post by: ninreznor on March 04, 2013, 08:48:38 AM
this is how it appears in the book.
O
"
CH3CH2CH2C-H
|
CH2CH2CH3
hope you can understand that.
so what i thought is that because the carbonyl group doesn't appear at the end of the chain but has an hydrogen attached to the carbon atom then it is an aldehyde.
longest chain of carbons is 6 so hexan but if the carbonyl isn't on the cahin then it must be a hexane ring
so what i have is cyclohexancarbaldehyde?
is this anywhere near close?
Post by: Hunter2 on March 04, 2013, 09:02:17 AM
Post by: billnotgatez on March 04, 2013, 09:43:46 AM
A GOOGLE search gives the possibilities
2 ethyl pentanoate
ethyl 2 methylpentanoate
2-ethyl-3- methylpentane
not
2-Ethyl-pentanale
And none of them appear to have just 1 oxygen
Post by: Hunter2 on March 04, 2013, 12:22:58 PM
Post by: ninreznor on March 04, 2013, 12:29:52 PM
O
"
CH3CH2CC-H
|
CH2CH2CH3
The only thing i can't understand is the -CHO group.
at first i thought it was 2-(1-methylethyl) butanal, but i keep coming up with different names and i'm not sure where to start the chain.
Post by: Hunter2 on March 04, 2013, 12:37:35 PM
"
CH3CH2CHC-H
|
CH2CH2CH3
That is the longest chain.
Post by: billnotgatez on March 04, 2013, 01:25:21 PM
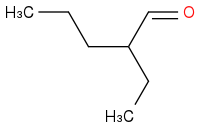
I tried this in SMILES
it does not quite look like the one on
http://www.chemspider.com/Chemical-Structure.81914.html
but it is very similar
Post by: Hunter2 on March 04, 2013, 01:43:55 PM
It is the same compound.
Post by: billnotgatez on March 04, 2013, 04:16:25 PM
the oxygen faces a different direction
but you are right baring the look it appears to be the same
This is my first post in SMILES
Post by: Borek on March 04, 2013, 05:35:08 PM
The 5 carbon backbone is not the same shape
the oxygen faces a different direction
You have to remember single bonds are not stiff, they rotate.