Post by: smatik on March 26, 2013, 07:49:36 AM
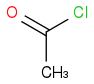 and
and 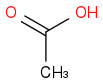 . I know that O is more electronegative than Cl and hence will increase the amount Δ+ on carbonyl carbon more than in the case of Cl also small OH molecule will provide less hinderance than large Cl. So according to me the answer should be
. I know that O is more electronegative than Cl and hence will increase the amount Δ+ on carbonyl carbon more than in the case of Cl also small OH molecule will provide less hinderance than large Cl. So according to me the answer should be  but the correct answer is
but the correct answer is  .Can you please explain me how? Thanks
.Can you please explain me how? Thanks
Post by: Sunil Simha on March 26, 2013, 08:12:43 AM
Post by: smatik on March 26, 2013, 08:22:21 AM
Post by: Sunil Simha on March 26, 2013, 08:30:58 AM
Post by: smatik on March 26, 2013, 08:50:08 AM
BUT Phenol is more ortho and para directing than chlorobenzene(Due to resonance) which means the Δ- charge developed in case of ch3cooh is more than that in case of ch3cocl and hence carbonyl carbon of ch3cocl becomes more favorite site for a nucleophillic attack. Thanks for the discussion..very useful and i got my answer
Post by: Sunil Simha on March 26, 2013, 10:01:01 AM