Post by: Enthalpy on March 30, 2015, 02:35:47 PM
Quadricyclane is an energetic hydrocarbon that some people would like to use as guess what - but I checked a few prices for norbornadiene and it seems expensive.
The synthesis would be, if I grasped it properly:
Cyclopentadiene from refineries and acetylene
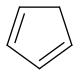
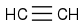
make norbornadiene by Diels-Alder
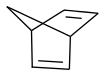
and by photoisomerization quadricyclane
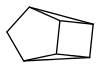
(the appended images may be clearer)
----------
Norbornadiene is offered at 120$/kg in 100kg amount on one site, but only 36€/0,25L by Merck a decade ago. Is that a realistic price in tons amounts?
What is expensive there? Is the reaction slow, dangerous...? Could it accelerate with a pair of chlorines or bromines at proper locations? On the cyclopentadiene or (hopefully not) on the acetylene?
----------
The photoisomerization looks rather affordable with a mercury lamp and a sensitizer, but its quantum efficiency is like 1%... As we have a choice of UV lamps now, powerful and at varied wavelengths, I vaguely hope that the proper one without a sensitizer would be more efficient.
Would you know a UV absorption spectrum for norbornadiene?
Thank you!
Post by: discodermolide on March 30, 2015, 07:40:36 PM
Cyclopentadiene itself is a dimer and has to be cracked to get the monomer. This involved heating the dimer, which undergoes a retro-Diels-Alder reaction to give the monomer which is then trapped with the new dienophile. Then you have to purify the entire mess.
Now I don't know if they are crystalline so it may require distillation.
All in all quite a tricky process.
Halogen substitution will probably slow the entire process down requiring higher temperatures for reaction.
Quadricyclane is a [2+2] cycloaddition, again quite reversible so difficult to isolate and keep intact. I don't know the best quantum yield that has been achieved.
These and other problems may well contribute to the high prices of these compounds.
Post by: Enthalpy on March 31, 2015, 11:31:23 AM
The dimer of cyclopentadiene is broken by heat, the monomer separated by distillation can be stored for a limited duration without having to combine it first.
Something like electrophile groups help Diels-Alder. No idea if this applies here with acetylene, nor if the groups must be on acetylene or on cyclopentadiene. But if electron-rich groups are better, just fine!
Under mercury UV and a sensitizer, the equilibrium between norbornadiene and quadricyclane is like 95% alkane versus 5% diene.
And meanwhile I've checked that 172nm from a xenon lamp fits all alkenes with separated double bonds. Heavy alkenes would accept also 193nm from an ArF lamp, but Xe lamps are better. Excitation of the pi bond happens essentially with every absorbed photon, but the story isn't finished then.
Post by: discodermolide on March 31, 2015, 11:54:51 AM
In your system you just have normal electron demand and you have several competing reactions: re-dimerisation of cyclopentadiene (a Diels-Alder reaction) and reaction with acetylene, also a Diels-Alder reaction as well as the thermal reversal of both. So there is nothing to stop the equlibrium forming, unless you remove one of the constituents. Norbornadiene can be nicely coordinated to transition metals such as Nickel.
I'm not a photochemical expert but 95% cyclised, even with that strain is a bit strange?
Post by: Enthalpy on March 31, 2015, 01:11:35 PM
Well, if somebody seriously wants to burn quadricyclane in an engine, it's a matter of 100t/month, so a simple setup can be taylored to produce it. Separating the much bigger cyclopentadiene dimer from the products and reinjecting it in the cracking unit looks accessible, doesn't it?
Meanwhile I've seen that usual Diels-Alder put a pair of -COOMe on the acetylene, so this must be where the electron-withdrawing group belongs. Conversely, would amines (dimethyl-) on the cyclopentadiene do the trick?
Reaction yield of the photoisomerization: I have obviously no opinion about it...
US Patent 6,635,152 shows 80% to ~100% depending on the sensitizer and Nbd's purity
Orgsyn stopped at 80%
I vaguely imagine that the photosensitizer acts on the alkene far better than on the alkane, and that changing from the Hg lamp to a Xe lamp without sensitizer may squander this advantage.
Post by: discodermolide on March 31, 2015, 02:32:22 PM
Amines on the dienophile also work well.
I would not really want to combust quadricyclane in my car engine. My car is so old it just manages to run with me in it never mind 100t of quadricyclane.
I vaguely remember that using a sensitizer reduces the energy of intersystem crossover thus reducing the energy required for the photochemical reaction, i.e. the alkene and suppressing side reactions?
Post by: Enthalpy on April 01, 2015, 08:40:05 AM
Others want to store sunlight in a norbornadiene, modified or catalyzed to absorb at visible wevelength, and let the quadricyclane release heat when needed.
But the engines I thought of are more thirsty. For instance the pretty-cute RD-170
http://www.lpre.de/energomash/RD-170/index.htm
whose four main chambers swallow each 433kg/s (1 bathtub) of already hot oxygen to burn 165kg/s of kerosene in D=380mm~L - because, to lift ten railway engines, this is what it takes.
To water such thirsty boys, one better has beverages decently easy to produce.
-----
Amines at the acetylene are just as good to burn as at the cyclopentadiene, and may lower the freezing point, fine.
Post by: Enthalpy on April 02, 2015, 09:45:47 AM
Would the appended synthesis of the N-cyclopropyl-7-aza-quadricyclane make any sense? Starting from butadiene or furane instead of cyclopropadiene dimer. This one keeps the interesting performance of quadricyclane.
Though, I'd like C+N~12. Is there a means to replace the -COOMe by an amine (without dihaloacetylene), instead of making a cyclopropylaza?
Post by: discodermolide on April 02, 2015, 10:53:45 AM
http://en.wikipedia.org/wiki/Paal–Knorr_synthesis (http://en.wikipedia.org/wiki/Paal–Knorr_synthesis)
The rest should be OK.
Post by: Enthalpy on April 03, 2015, 08:02:59 PM
Post by: Enthalpy on April 14, 2015, 04:41:21 PM
As haloacetylene has drawbacks, maybe 1,2-di- or trichloroethylene can provide a halonorbornene, where a double bond would be restored and azetidine be coupled? I imagine chlorine helps the Diels-Alder step.
Though, I fear annoyances in both cases (drawings appended):
- From dichloronorbornene, abstracting HCl would create the double bond towards the tertiary carbon, according to Zaitsev;
- From trichloronorbornene, abstraction of Cl2 seems forbidden because the chlorine aren't anticoplanar.
- (Or maybe the attempt makes no sense for other reasons...)
What do you think? Thanks!
Post by: discodermolide on April 14, 2015, 08:51:56 PM
Post by: Enthalpy on April 15, 2015, 05:12:33 AM
So instead, first dichloroazetidylethylene, then Diels-Alder to he substituted norbornene?
What about the CL2 elimination: can it proceed despite the C that hold the Cl can't rotate?
Post by: discodermolide on April 15, 2015, 06:04:50 AM
You could try a Pd catalysed coupling of the vinyl chloride with the azetidine. Usually this works well for aromatic halides (Buchwald-Hartwig). In this case it may well have a chance. See for example
A new reactivity pattern for vinyl bromides: cine-substitution via palladium catalysed C-N coupling/Michael addition reactions
M. C. Willis, J. Chauhan, W. G. Whittinham, Org. Biomol. Chem., 2005, 3, 3094-3095.
and
(t-Bu)2PNP(i-BuNCH2CH2)3N: New Efficient Ligand for Palladium-Catalyzed C-N Couplings of Aryl and Heteroaryl Bromides and Chlorides and for Vinyl Bromides at Room Temperature
Ch. V. Reddy, J. V. Kingston, J. G. Verkade, J. Org. Chem., 2008, 73, 3047-3062.
Post by: Enthalpy on April 16, 2015, 09:56:54 AM
Found the papers' abstracts, so apparently routes exist.
You wrote "HCl elimination": do you consider it could create a double bond at the proper location? I thought the double bond would appear towards the tertiary carbon where I don"t need it, that's why I considered the trichloro.
Post by: discodermolide on April 16, 2015, 12:58:29 PM
Post by: curiouscat on April 16, 2015, 03:12:56 PM
Quote
Norbornadiene is offered at 120$/kg in 100kg amount on one site,
Which site?
Post by: Enthalpy on April 17, 2015, 05:31:04 PM
If you have the vicinal dichloride then it does not matter as the molecule is symmetrical. The worst thing that could happen is di-substitution!
But would the subsequent step, photoisomerization to quadricyclane, work if the new double bond is towards the tertiary carbon? I believe to understand the photo step as a 2+2 cycloaddition.
Post by: Enthalpy on April 17, 2015, 05:35:30 PM
Maya Biotech.QuoteNorbornadiene is offered at 120$/kg in 100kg amount on one site,Which site?
Such non-binding figures are little reliable, but the stone-old Chemdat CD from Merck wants 36€/0.25L, so a >100kg supplier could indeed bring the price to that area.
Post by: discodermolide on April 17, 2015, 10:54:27 PM
If you have the vicinal dichloride then it does not matter as the molecule is symmetrical. The worst thing that could happen is di-substitution!
But would the subsequent step, photoisomerization to quadricyclane, work if the new double bond is towards the tertiary carbon? I believe to understand the photo step as a 2+2 cycloaddition.
I shall try the SMILES to see if I can explain myself better:
Start with this compound:
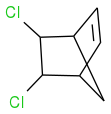
then make this one
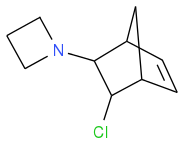
finally eliminate HCl:
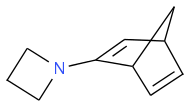
then do the 2+2 to give:
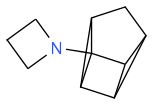
The only problem I can see is the formation of
as a side product, which may happen but might sterically be less likely and I believe you could control the conditions to avoid much of this forming.
Post by: curiouscat on April 18, 2015, 02:50:29 AM
Maya Biotech.QuoteNorbornadiene is offered at 120$/kg in 100kg amount on one site,Which site?
I had a look at Maya Biotech & somehow I'm skeptical that they have ever come within miles of Norbornadiene.
Post by: pgk on April 18, 2015, 01:48:11 PM
http://cdn.intechopen.com/pdfs-wm/34620.pdf
See also, the attached file.
Post by: Enthalpy on April 20, 2015, 01:02:46 PM
http://www.uspkhim.ru/php/getFT.phtml?jrnid=rc&paperid=745&year_id=2002
but the language takes me some time to guess where is what information. Nice: they detail for several subtituant groups, including dimethylamine, which coud represent azetidine.
Curiouscat: I won't risk any kind of opinion on the topic and gladly follow your opinion. 400€/0.5L at Sigma-Aldrich, does it make 120$/kg elsewhere credible in 100kg?
Discodermolide: I had imagined from Zaitsev (misunderstood?) that the HCl elimination from
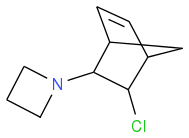
would rather put the double bond at
where the photo step looks compromised. Unless some trick exists?
Two azetidyls in a byproduct would raise the boiling point enough to be separated if any worrying. Or let it polymerize for removal maybe.
Post by: discodermolide on April 20, 2015, 01:20:59 PM
Post by: Enthalpy on April 20, 2015, 01:33:56 PM
Then, this is a very serious fuel candidate. Not the top-magic in the list, but an excellent one maybe accessible to classical production. Far above the Boctane that tempts designers presently.
Cyclopentadiene is cheap, dichloroethylene too. Azetidine costs a bit but is obtained from thermal elimination of NH3 from cheap 1,3-diaminopropane with HCl, so home production could be cheap.
If the reaction steps can be made the big way at a reasonable speed (I hope chlorine helps Diels-Alder), just scale to tons.
I'll re-check a heat of formation and performance. Then, the melting point, boiling point, stability... must be experimented.
Post by: Enthalpy on April 21, 2015, 11:41:52 AM
The path through dichloronorbornene being clearly the better one, I've appended an updated synthesis scheme.
----------
Wanting a single azetidyl on norbornene, how to avoid a second one...
The opinion of AM1 is that the second condensation is almost as favourable as the first one, just 12kJ less good, without any abvious collision. Though, this is for the reaction's products - no idea about the intermediates, the approach conditions and so on.
More promising: Mpbpvp estimates atmospheric boiling points as
+191°C Dichloronorbornene
+240°C Azetidylchloronorbornene
+284°C Diazetidylnorbornene
and azetidine boils at +53°C.
If the amine-chloro condensation can happen in gas phase (can it?) the reactor would just contain an excess of dichloronorbornene slightly above its boiling point, introduce azetidine slowly, and let azetidylchloro- rain down to collect it so it doesn't react further. If necessary (49K contrast in boiling points), the reactor's bottom could be a packed distillation column, to better separate azetidylchloro- from dichloro-. If any diazetidyl- forms, later distillation can separate it.
Post by: discodermolide on April 21, 2015, 02:57:26 PM
Perhaps a continuous flow system would be better to reduce the contact time with the temperature zone.
I think this may require some experimentation to get right, but conditions could very probably be found.
Post by: Enthalpy on April 22, 2015, 02:35:52 PM
----------
If aminating in liquid phase instead of gaseous, could some alkaline metal be first dissolved/reacted in the azetidine, as is done with ammonia and aryls, to kick the reaction? ...or would the reaction kick back maybe...? ;D
----------
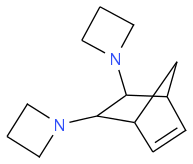
I've hand-estimated the heat of formation of the azetidyl-, as well as dimethylamino- and bis(dimethylamino)-quadricyclane. At +417, +329 and +356 kJ/mol (298K liquids), they would all bring 5s more than RG-1, just one second less than the flammable parent quadricyclane, so the flash point, melting point and others can decide - mixes being often better.
All aziridines seem toxic, alas. The bis(dimethylamino) would start from trichloroethylene, at least in my imagination. Dimethylamines takes a bit more oxygen.
Marc Schaefer, aka Enthalpy
Post by: discodermolide on April 22, 2015, 03:38:20 PM
Aziridines, I think you can forget due to the toxicity. The dimethylamino substitution should be ok. In either case the synthesis would be the same as previously discussed.
Post by: Enthalpy on April 23, 2015, 03:38:26 AM
So I just hoped to mimick that with KNC3H6 with a swift reaction at room temperature.
OK, I definitely give up aziridines, as you confirm the impression. And all three-membered aminated cycles and polycycles, alas.
Having azetidyl-, methylamino- and bis(dimethylamino)- quadricyclane as candidates gives some hope that one or a mix will survive the many conditions for a fuel.
Post by: discodermolide on April 23, 2015, 05:41:03 AM
Deprotonation is better achieved with NaH or similar. Dissolving metals mean solvated electrons and they may well cause havoc in this system.
Post by: Enthalpy on April 26, 2015, 02:56:20 PM
Atmospheric boiling points in °C as estimated (!) by Mpbpvp, route to dimethylamino-quadricyclane:
+191 Dichloronorbornene
+207 Chloro-(dimethylamino)-norbornene (harvest it)
+224 Bis(dimethylamino)-norbornene (avoid it)
Again Mpbpvp estimates, route to bis(dimethylamino)-quadricyclane:
+201 Trichloronorbornene
+228 Dichloro-dimethylamino-norbornene
+243 Chloro-bis(dimethylamino)-norbornene (harvest it)
+267 tri(dimethylamino)-norbornene (avoid it)
The differences are smaller than with azetidine and need some efficient distillation method. A lower pressure linders the temperature.
In addition to cyclopentadiene and di or trichloroethylene, dimethylamine is a mass product too. This fuel only needs to work.
Post by: curiouscat on April 27, 2015, 12:48:18 AM
How accurate are these Boiling Points you are mentioning?
Post by: Enthalpy on April 27, 2015, 10:00:45 AM
http://www.epa.gov/oppt/exposure/pubs/episuitedl.htm
from which, after installation, Mpbpnt.exe can be started directly. Though, installing just the small Mpbp from the package needs light touch - something like a dll or a knowledge file not in the Mpbp subdirectory.
It gets Smiles as an input, has a data base of known compounds and also estimates properties. Bp use fairly accurate except for quadricyclane, mp are completely unusable. It's just Stein&Brown and Joback - but additive methods consistently fail on mp, like 50K wrong.
Meas Pred Compound
+81 +90 Cyclohexane
+145 +147 Styrene
+246 +262 2,4,6-trichlorophenol
Not too bad. The vapour pressure must be about as accurate.
Post by: curiouscat on April 28, 2015, 02:15:39 AM
Post by: Enthalpy on March 26, 2016, 02:41:09 PM
Its fractional distillation, which could use a packed column, condenses the desired compound soon when it forms to harvest it. With a different pressure and possibly temperature, in the same reactor at a different time if desired, as well trichloronorbornene can become chloro-bis-dimethylamino-norbornene, or the amine can be azetidine - this isn't even limited to the present set of compounds.
Chloro-bis(dimethylamino)-norbornene can also result from adequate dibromochloronorbornene, which is expected to suppress the unwanted gem-bis-dimethylamino.
Not represented: the reactor needs cooling and a means to remove HCl. Or does HCl form dimethylammonium chloride here? That would be a reason to separate the distillation column from the reaction zone and collect the salt at the bottom or at a whirl.
Marc Schaefer, aka Enthalpy
Post by: Enthalpy on March 27, 2016, 07:02:32 PM
It needs a sensitizer, and many have been studied. Substituted norbornadienes tend to accept longer waves.
Acetophenone and plain norbornadiene to quadricyclane show 90% quantum efficiency at 366nm and 388nm:
Crc Handbook of Photochemistry and Photobiology, table 17.1 (Google book)
so for instance azetidylquadricyclane (C10H13N 147.22 g/mol) would need 7.5 mol/kg photons at 385nm (3.22eV = 311kJ/mol).
AlGaN diodes are 36% power efficient and produce 5W light at 385nm, while 405nm would be even better:
http://www.nichia.co.jp/en/product/uvled.html
so 1kg azetidylquadricyclane needs 6.5MJ electricity. At 100€/MWh = 28€/GJ, electricity costs 0.2€/kg or 40k€/200t only.
A production capacity of 200t in 20d*13h or 0.2kg/s needs 500kW light. If the diodes cost 1€/W the investment is reasonable. The claimed life expectancy of 50,000h lets 1W light produce 77kg azetidylquadricyclane, adding 0.01€/kg.
According to
http://www.nist.gov/srd/upload/jpcrd322.pdf (broad compilation, thanks Nist)
https://www3.nd.edu/~ndrlrcdc/Compilations/Tta/Tta0008.htm (acetophenone)
acetophenone extinguishes 2100 L/mol/cm at 406nm, so 1% in quadricyclane attenuates by exp(1) in 70µm.
With diodes that emit 5W and occupy 1cm2, a sketch for a 500kW UV reactor is appended :
- h500mm * D38mm plungers can contain 8*50 diodes to emit 2kW and dissipate 4kW.
- Water would flow at 0.4m/s, but prefer phytane and similar as they insulate
http://www.chemicalforums.com/index.php?topic=56069 - 48Vdc suffice in the plungers but are uncomfortable elsewhere.
- 250 plungers on a 40mm raster make the reactor 0.5m*1.1m*0.6m.
Marc Schaefer, aka Enthalpy