Post by: insertwittyname on October 21, 2017, 06:51:37 PM
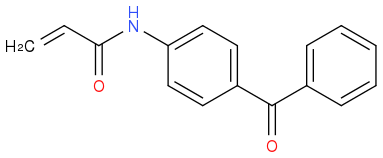 by a reaction between
by a reaction between 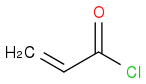 and
and 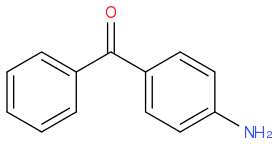 . I then took the 1H-NMR in CDCl3. While I've managed to find the peaks corresponding to the various protons, I can't see the one for the proton on N in the amide group. Mestrenova predicts the missing peak at a δ value of 8.84, but the only unidentified peak I have (which might be proton I'm looking for since all other peaks have been assigned) is at a δ=5.23
. I then took the 1H-NMR in CDCl3. While I've managed to find the peaks corresponding to the various protons, I can't see the one for the proton on N in the amide group. Mestrenova predicts the missing peak at a δ value of 8.84, but the only unidentified peak I have (which might be proton I'm looking for since all other peaks have been assigned) is at a δ=5.23However on integrating the protons, it only shows 0.15 protons, which makes me doubt if it really is the amide peak. Would be glad to hear any suggestions.
PS: I used triethylamine to neutralise the HCl formed. Could the basic nature of the amine abstract the amide proton, thus returning a value much less than expected?
Post by: Flatbutterfly on October 21, 2017, 06:59:19 PM
Post by: wildfyr on October 21, 2017, 10:59:37 PM
Post by: insertwittyname on October 22, 2017, 04:01:33 AM
Post by: kriggy on October 22, 2017, 08:14:53 AM
Post by: Flatbutterfly on October 22, 2017, 10:24:18 AM
Post by: clarkstill on November 16, 2017, 11:20:43 AM