I'm just a beginner in chemistry, but in my country there is this test which looks very much like the SAT, so perhaps I can help you somehow.
These facts are usually shown in an inorganic chemistry book. The compounds of glass (which is related to sand), the compounds of granite (which is not homogeneous), and the compounds of cement are important to know.
The structural formula of SiO2 is important to know too. It is certainly not just O = Si = O. It looks like this:
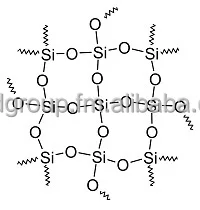
As for the precipitate colors, what exactly are you struggling with?