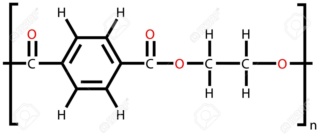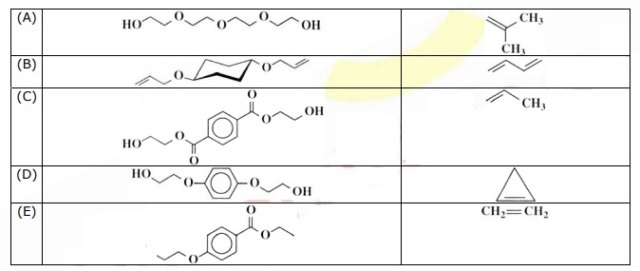
Hello. I just began studying polymers, and I was given this exercise that asks me to mark the option with monomers that produce polyester and polypropylene.
The polypropylene is actually very easy, but my problem is with the polyester. Isn't a polyester made from an acid and an alcohol--for example, terephthalic acid and ethylene-glycol? Then what even is that on option (C)? That doesn't look like an acid to me; that looks like an ester.
Could you guys please explain it to me?
Thanks
EDIT: perhaps I confused myself with the meaning of "monomer"


but I'm still confused with the formula. So I have to get the monomer and take out one OH and one H from the opposite side? Where does the other "ethylene-glycol" part go???!?!?!

I searched the internet and found this: