The following graph represents N
2 + O
2 <------> 2 NO
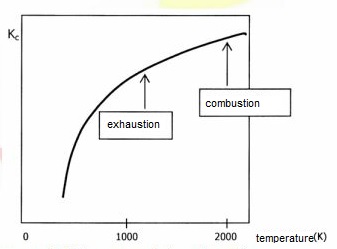
Which of the following propositions are true?
I) in the combustion chamber, the formation of NO is more efficient than in the exhaustion system.
II) the formation reaction of NO is exothermic;
III) lowering the temperature favors the decomposition of NO into N
2 and O
2.
Answer: (I) and (III)
Could you guys give me a hint here? I think that (I) is true because Kc = products / reagents, so the greater is the Kc, the greater is the quantity of products. This also helps me find that (III) is true. But what about (II)?
Thanks