Not sure if this is in the right section, as it's a problem I've been given to prepare for Olympiads, but I figured I'd try here first.
Disclaimer that I have absolutely no idea how to start here. Here's the problem:
For titration of a 25mL solution containing a mixture of H2SO4 and H3PO4, 37.2 mL of NaOH with a concentration of 1.008M have been used in the presence of a methyl orange indicator. The same volume of the solution required 49.6mL of NaOH with the same concentration for titration in the presence of phenolphthalein indicator. Calculate the concentrations of H2SO4 and H3PO4 in the solution.
The titration curves for both polyprotic acids are also given:
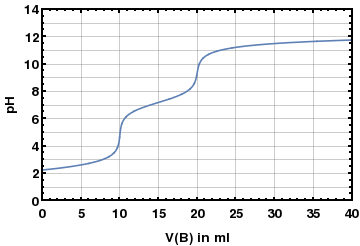

If anyone has any idea how to do this, please help.