Alkanes with wide liquid range:
Initially, I wanted a rocket fuel not easily flammable, hence boiling at +180°C and more, that wouldn't freeze on Mars, at possibly -100°C. Something like farnesane or phytane
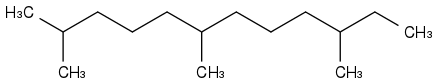
or
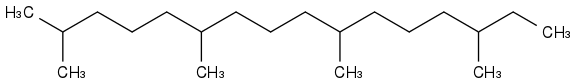
and then I realised that
many commercial uses have the same needs: transformer oil, lubricants, vacuum oil and grease, cooling liquid, hydraulic fluid including for aeroplanes, corrosion protection, oil for music instruments, fridge ice
http://www.chemicalforums.com/index.php?topic=56069.msg246917#msg246917 and next messages
and just
knowing a set of good compounds and having more measured melting points would be very nice. Presently, Mankind seems to have less than 50 cold melting points for branched alkanes, that's ludicrous to build a theory or make predictions.
The synthesis of these two compounds looks uneasy, maybe by oligomerisation of isoprene or myrcene
http://www.chemicalforums.com/index.php?topic=56069.msg297847#msg297847One young company, Amyris, goes the biology route
http://www.chemicalforums.com/index.php?topic=56069.msg203568#msg203568Silanes may fit many uses but not fuels
http://www.chemicalforums.com/index.php?topic=56069.msg327456#msg327456I believe these routes are inaccessible to pre-university students. Some alternatives follow here.
Check what Nature has already done. From much light or heavy Diesel oil, extract the hydrocarbons, saturate all bonds or eliminate unsaturated compounds, to have only alkanes. Freeze and distil successively to keep the compounds with exceptional liquid range. Separate every compound as you can, identify it, measure the melting and boiling points, and as you're there, the density, viscosity and so on. If the yield is decent, sell the interesting compounds extracted from oil. If not, maybe someone will synthesize them. Search for some general rules about the melting point, but that's non-trivial, and more examples are needed to eliminate wrong theories; that will help design better molecules.
Synthesize many alkanes at once, then separate the good ones as previously. This may need Grignard reagents which are dangerous, so ask chemists here and at the University. By reacting A
1...A
100 with B
1...B
100, you get about 10 000 AB compounds from which some can be interesting.
Synthesize the amine homologues, tertiary, saturated and branched.
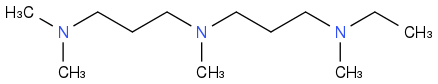
Much easier than alkanes, little more efficient as rocket fuels, less good in other uses, hence more difficult to sell.
http://www.chemicalforums.com/index.php?topic=56069.msg254340#msg254340I suppose these are well within your reach, without big surprises
http://www.chemicalforums.com/index.php?topic=56069.msg272080#msg272080you can even buy the propylene amines and methylate them. And I have nothing against ethylene amines, just propylene should resist cold better. Other amines could be good, say bytulhexyloctylamine, or sisters of propylamines with longer branches. Simple nice syntheses, the useful part is (a) how easy production is (b) what the physical properties are: melting point, flash point, viscosity, density... and odour is interesting too.
As a variant, synthesize a bunch of
alkyl-alkyl'-alkyl"-amines from a mixture or alkenes, haloalkanes or alcohols, then separate and identify the fuels with the best liquid range, search for azeotropes, check how much separation is useful in mass production. This could be cheap enough for cars and make use of C
3 and C
4 hydrocarbons that are presently torched at the gas and oil wells.
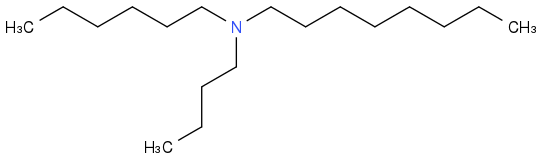
The same operation with silanes would open different markets, but seems too dangerous for pre-University students.
Synthesize farnesane from caparrapi oil which is available from a tree in South America, just like we have rubber from hevea. Or start from farnesol, equivalent but less demonstrative. That synthesis of farnesane seems much easier: separate, saturate, dehydrate, saturate again, purify.
From
http://www.chemicalforums.com/index.php?topic=56069.msg338107#msg338107to
https://www.chemicalforums.com/index.php?topic=56069.msg349796#msg349796It involves hydrogen (I believe alternatives exist) and maybe special apparatus. The project would check if farnesane is obtained, how easy the process is, what unexpected problems arise. If farnesane gets as cheap as natural rubber, it won't replace kerosene for airliners, but it can feed rockets very easily, maybe cool computers, and so on. I expect many
commercial uses. That process would compete against Amyris' biological route.
Here you can make chemical syntheses. Uncertainties are small. Most results can be published. I'm not a chemist, so
I hope you'll get advice here and at your University.