The question is from INChO - Indian National Chemistry Olympiad. I'm typing the question as such first.... I'll show my working also
I just want to know if I'm correct....
4.8 Draw the structure of dimethyl 1,2-cyclobutene dicarboxylate (J)When
J is heated with maleic anhydride(butenedioic anhydride), an unusual reaction takes place to form compound
K. When
K is boiled with aq. NaOH and the solution is acidified, compound
L (C
10H
10O
8), which is optically inactive, is obtained. 1.0g of L reacts with 77.5mL of 0.2M NaOH.
4.9 Equivalent weight of compound L is _________4.10 The no. of -COOH groups present in compound L is ________4.11 L is expected to contain (Mark X for all the appropriate choices)
(i) Cyclobutane ring ___
(ii) Cyclohexane ring ___
(iii) open chain structure ___
(iv) one double bond ___4.12 Draw the possible structure/s of compound L and compound K.4.13 Draw the structure of a possible intermediate in the reaction.L on heating forms
M (C
10H
6O
6) which on reaction with
N gives a polymer
O.
 4.14 Draw the structure of a representative segment of polymer O
4.14 Draw the structure of a representative segment of polymer O.
MY APPROACH:
4.8 That's pretty easy

. This is my structure..
 4.9
4.9 As 1.0g of L reacts with (77.5)(0.2) m.eq. of NaOH, equivalent weight is 1000/(15.5) ~64.5 g/eq
4.10 no. of -COOH groups in L = (Molecular Wt.)
/(Eq. Weight) = 4
4.11 Well for this question I've calculated DBE(Double bond equivalent) of L and got '6'. 4 of the 'double bonds' are with 4 -COOH s.
So that leaves L with 2 more unsaturation units .
I expect it may contain a ring (hopefully a cyclohexane ring) and 'may' be a double bond.
4.12Since I've expected L to contain cyclo hexane ring and a double bond, I think the possible reaction in accordance with it is
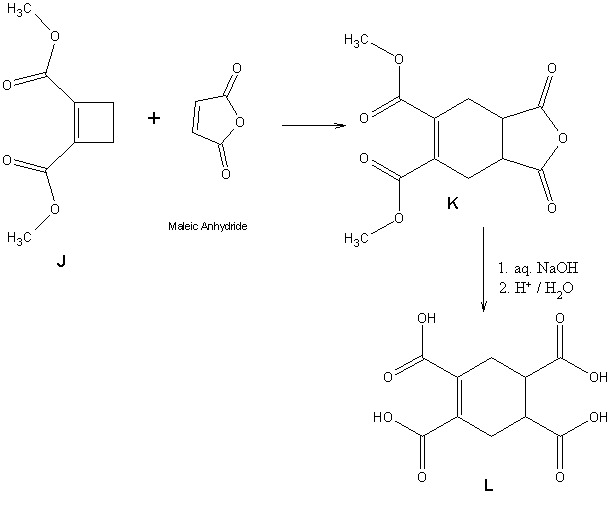 4.13
4.13 No idea how the reaction proceeds.. Is it by free-radical mechanism or ...
 4.14
4.14I have some vague idea about this but first let's agree upon 'L'.
But there are other approaches also which yield different products..
I'll post them later..