I have a question about an equilibrium example in my textbook that I believe is giving the incorrect answer.
Q: The Haber Process is the industrial method for the production of ammonia: 3H
2(g) + N
2(g) 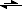
2NH
3(g). If the pressure in a vessel containing hydrogen gas and nitrogen gas is increased, how is the Haber process affected?
A) It is favored
B) It is disfavored
C) It is unaffected
D) It is impossible to tell from the given information
My book says that A) is the answer since an increase in pressure will cause the system to shift to the right where there are fewer moles of gas to compensate for the pressure.
However, in my opinion it should be D) since without extra information you can't tell if the pressure increase is from increase in volume or through the addition of inert gas to the system.
Can anyone shed some light on this?