Hello,
This is my first post on this site, so nice to meet you all.
I took an H-NMR spectrum of
trans-Methyl cinnamate the other week, and upon examining it, there were two peaks that I just can't account for.
Here is the spectrum:
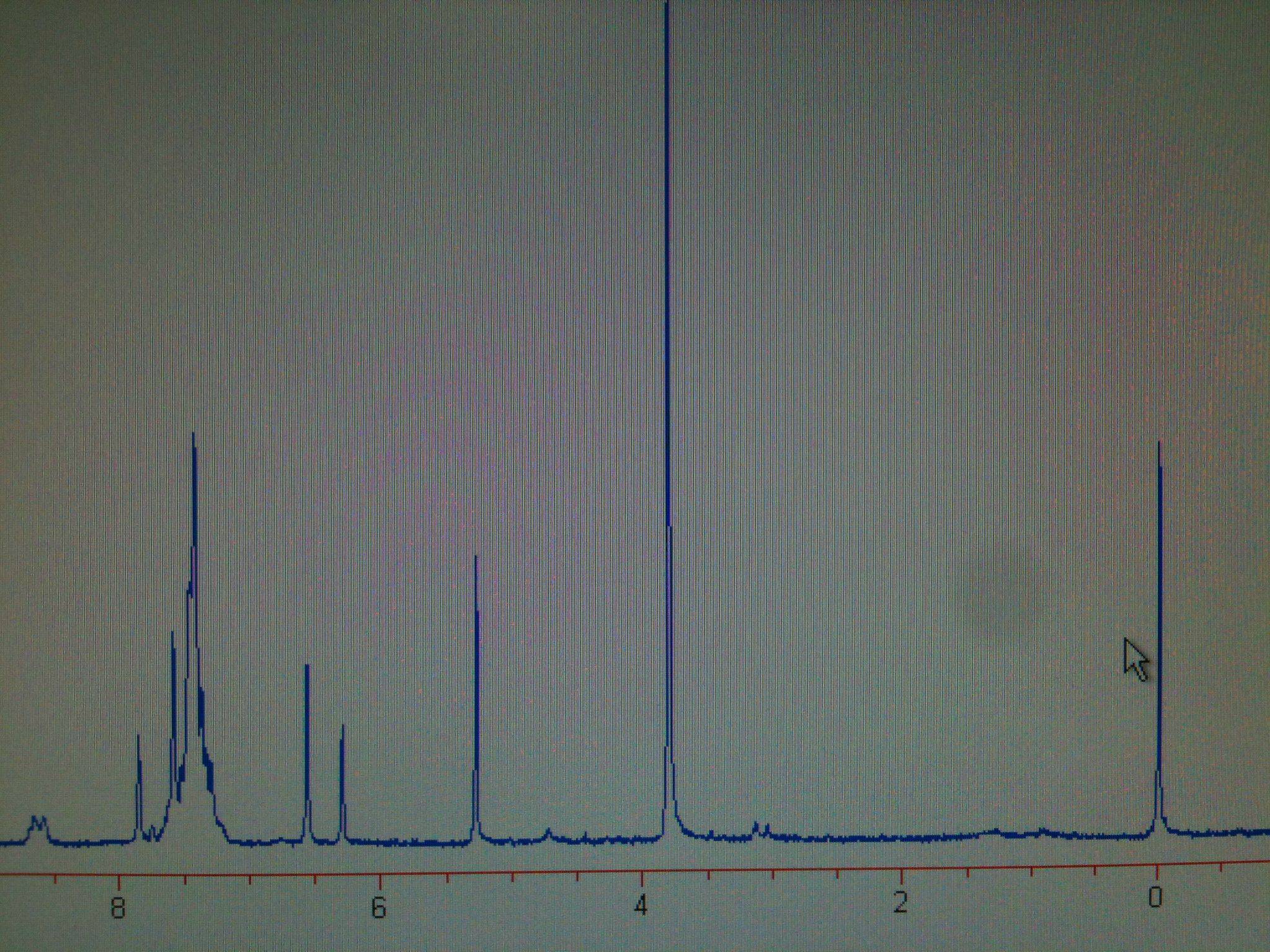
This is my data so far:
3.80ppm RCOOC
H36.45ppm R=C
HCOOR
7.29-7.65ppm C
6H5R
7.69ppm PhC
H=R
However, I can't account for the peaks about 5.25ppm and 8.35ppm.
My procedure:
1. refluxed cinnamic acid with SOCl
2 to form cinnamoyl chloride.
2. Removed excess SOCl
2 via distillation and 2 washes of hexane, which was also distilled off.
3. refluxed cinnamoyl acid in CH
2Cl
2 with pyridine and methanol.
4. Separated with NaCHO
3 in separatory funnel into anhydrous CaCl
2.
5. removed excess CH
2Cl
2 via distillation.
So, what caused the peaks about 5.25ppm and 8.35ppm?
At first, I assumed it could be pyridine, due to the 8.35 peak, but I crossed that one off the list
Any help would be fantastic,
Thank you,
Cryptonic