What happens if you mix a primary amine with an aldehyde:
primary amine + aldehyde
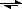
?
How will this product ? interact with an enolate.
I assume an enolate forms as well, both look like electrophiles to me
An enolate is not an electrophile - the fact that it is anionic should immediately tell you that.
Note: This first step is a very classical named reaction.