If we were to look at an electronegativity chart we would see that H is 2.1, O is 3.5, and F is 4.0
We also know that:
A compound is ionic if and only if ΔEN > 2.0
A compound is polar covalent if and only if .5 < ΔEN < 1.7
A compound is non-polar covalent if and only if ΔEN < .5
Yet there is a small gap between 1.7 and 2.0. The rule for this gap is very important to acidity; if a metal is involved in the compound, then the compound is said to be ionic—if not then polar covalent.
HO bonds have a ΔEN = 1.4 so they follow rule number two, but HF is 1.9; so strong, in fact, that it is on the brink of being an ionic substance. Therefore, HF is a much stronger acid compared to H
2O because of its much greater capability to attract the electron from the hydrogen.
Yet, because F
- can also act as a Lewis base shown by the following formula:
F
- + H
2O
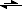
HF + OH
-The acid can, in effect, partially cancel itself out slightly.
Furthermore, HF is capable of strong intermolecular forces: hydrogen bonding; this characteristic inhibits complete theoretical disassociation.
If we were to move down a group to Chlorine then we can see that Cl EN = 3.0, therefore HCl ΔEN = .9, which means it is a polar covalent compound; thus, because the bond between H
+ and Cl
- is much weaker (but still strong compared to a non-polar covalent acid) than that in H
+ and F
- then the aforementioned oddities with HF no longer occur. For example, Cl
- will not act as a Lewis base and attract a H
+ from water.
Cl
- + H
2O

No reaction
Similarly, HCl does not qualify for hydrogen bonding, therefore making it favorable for the molecule to completely disassociate.