I am trying to understand why higher concentrations of H
+ speeds up cyanide action.
As far as I know, what kills the individual is the cyanide anion (
CN-), not the cyanide acid (HCN).
Let's say we have a cyanide salt dissolved in a glass of water and then we add an acid (or we can imagine an empty stomach that is extra acidified):
1-) KCN
(aq) 
K
+(aq) + CN
-(aq)2-) CN
-(aq) + H
2O
(l) 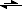
HCN
(aq) + OH
-(aq) (hydrolysis)
3-) OH
-(aq) + H
+(aq) 
H
2O
(l)Global process: KCN
(aq) + H
+(aq) 
HCN
(aq) + K
+(aq)As we can see, the addition of ions H
+ reduces the ammount of ions CN
- and favours the formation of HCN. What I am missing?
Another ilustration: In gas chambers, one makes the following reaction, to produce HCN
(g):
H
2SO
4 + 2KCN

2HCN + K
2SO
4Why not ingest KCN
(aq)?
Obs1: Sorry for my english
Obs2: I am not trying to kill anyone, just trying to understand the process.