ref. 1): "acidity" is not well defined in non-aqueous "solutions" ( i.e. V
2O
5 in molten Al
2Cl
6 as "solvent")
.. so I don't see how something not defined might be influenced here
(furthermore, it is quite difficult to have a melt of Al
2Cl
6, as it usually sublimes before it melts)
ref. what happens, two potential reactions come to mind:
- V
2O
5 being a strong oxidizer, it might well produce some chlorine initially at elevated temperatures, for example:
V
(+V) + 2 Cl
- 
V
(+III) + Cl
2V
2O
5 + 4 Cl
- 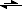
2 VO
2- + 2 Cl
2 + O
2-(amongst other redox-processes)
the oxygen-dianions stepwise will transform Al
2Cl
6 to Al
2O
3 + 6 Cl
-- this chlorine, however, might transform V
2O
5 to VOCl
3 , which is voilatile under these conditions (b.p. 127 °C )
6 Cl
2 + 2 V
2O
5 
4 VOCl
3 
+ 3 O
2the oxygens produced thereby in turn might re-oxidize the V(+III), so my total process expectation would be
2 Al
2Cl
6 + 2 V
2O
5 
4 VOCl
3 
+ 2 Al
2O
3... but I wouldn't bet the ranch on that
ref order of lewis acid strength:
take a look at what kind of inductive effects the different substituents are known to have
the less electron density remains at the boron, the more strong a lewis-acid it will become
regards
Ingo