The letters are not arbitrary. They stand iso and tertiary and represent isomers of the parent compound.
So you have propanol, a straight chain and iso-propanol where you now have the OH being attached to the middle carbon like this:

and iso
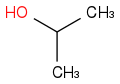
See if you can draw all the isomers for butanol.