in addition:
though KI
3 sporting the I
3- anion is most often named when the solubility of iodine in KI-solutions is discussed, this by no means is the only species involved here.
In general, a bunch of anions [I
2n+1]
- is observed, and at times quite long chains are formed (you might compare this to polysulfide-dianions)
the longer the
linear part of the chain, the darker and the more intense the colour becomes, and with forced "long" linearity (like, for example, when incorporated to starch helices) even minor traces of polyiodine-anions (hence, indirectly, iodine) might become very intense, and hence detected.
(that's why we use starch in iodometry)
with no KI around, the colour results from (in neutral environment: weak) autodisproportionation of iodine in water: I
2 + H
2O
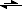
HIO + HI

HIO + I
- + H
+(with further processes like 5 HIO

2 I
2 + 2 H
2O + HIO
3 )
the iodine thus formed again will be involved with minor polyiodid-anion formation
that's why "solutions" of iodine in water appear to be slightly brownish-yellow
regards
Ingo