Fun.
Specific impulse, for rocket propellants, yes. And because hydrogen atoms are so efficient in a fuel, any added -C bond must add very much strain energy to justify itself. Cyclobutanes bring a little bit, cyclopropanes are interesting, and odd strained compounds get interesting as compared with so-called "kerosene" (RP-1) - but provided they're safe and cheap enough and so on, that's why we still have kerosene.
No miracle launcher fuel has spread since decades, because most are too dangerous, or perform worse than kerosene, or cost too much - and the next step is hydrogen, for which engines exist already. So what can improve is (1) safety, especially by replacing hydrazine (2) liquid range, to take-off from Mars (3) maybe perhaps the performance.
By the way,
how stable is bicyclobutane?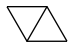
I've only found "enough to be isolated", which isn't accurate enough to pour 200t in a rocket... Even nitromethane (seducing as a monopropellant) is excluded as it detonates after a 100m fall. So is bicyclobutane stable at shocks, like a drop on concrete from 100m? At heat, say +200°C? Because, if a fuel can't cool the engine by flowing in the jacket, then we can burn ethylene, which is available and extremely efficient.
One difficulty with "energetic materials" is that the name mixes different uses that have opposite requirements. Explosives have the oxidizer in the same molecule, cruise missiles want volume-effective fuels burnt with air producing a small signature, liquid launchers want safe mass-effective fuels burnt with oxygen and care very little about
density. Most proposed launcher fuels, with oxygen atoms, multiple bonds, or unstrained cycles, pursue wrong objectives.
Among the
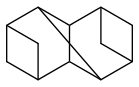
very few fuels that might be useable in liquid rockets and outperform kerosene and methane or cyclopropane are some cubane derivatives if mass-produced, some spiro compounds, maybe stellane (how efficient?)
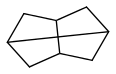
widely described in
http://www.tdr.cesca.es/TESIS_UB/AVAILABLE/TDX-0618107-110904/CAR_Tesi.pdfladderanes are no miracle
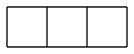
diasterane and triasterane would probably be good if mass-produced
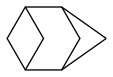
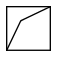
bicyclo[1.1.1]pentane is efficient and said to be stable
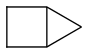
housane is efficient
Most of these (not exhaustive list!) should rather be coupled in pairs or trios (but this wastes performance) to be liquid, and tertiary amine compounds are welcome.
The
amine option has been investigated too little up to now. Aza brings performance and eases syntheses.
Photosynthesis as well, enabled by the now available excimer lamps, whose purchase and electricity costs are affordable by a launcher, should be investigated.