Pi bonding refers to sidewise overlapping of orbitals . Therefore d-pi- p-pi refers to when one d orbital and p orbital of another atom overlap sidewise to form a pi bond .
Yes phosphorus shows dΠ - pΠ in POCl
3 or similar species .
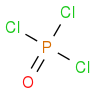
POCl
3 is a sp
3 hybridized , so 1 s and 3 p orbitals will used for making sigma bonds . But there is still one pi bond between P and O , So d orbital of phosphorus will be used for sidewise overlapping with an p orbital of Oxygen .
i hope you have understood .
