So I graduated in chemistry and am helping my roommate with his Ochem homework, and we run across this question:
1-iodo-but-2-ene reacts with sodium methoxide to form what product? (or, in structural terms
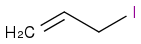
+ NaOCH
3). I think that the methoxide group will attack the beta carbon's hydrogen in an acid-base form and cause the elimination of the iodine, creating CH
2CCH
2. He thinks it will undergo an SN
2 reaction and form
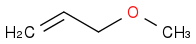
. The problem is that his
feels more right, but I don't have a reason, as I have always been told that acid-base chemistry happens the fastest of all. Help?