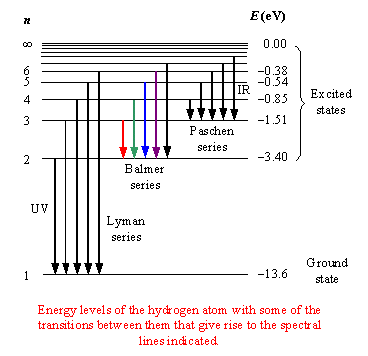
I am using the equation E=hc/λ to calculate the photon energy for each wavelength in Hydrogen spectral series.
What I found was that energy required for electrons to jump to a particular level differed for Lyman, Balmer and Paschen series.
For example, energy required to transition to n4 are 2.048x10
-18 joules (Lyman), 4.577x10
-19 joules (Balmer) and 1.059x10
-19 joules (Paschen).
My questions are:
- Can you explain to me why energy required to transition to the same levels differed for each experiment?
- I know Lyman's experiment was conducted in a vacuum and Balmer/Paschen in air, so how does a vacuum effect the required energy for electrons to transition states?
- Did temperature also play a part in the differences in these experiments?
Thanks.