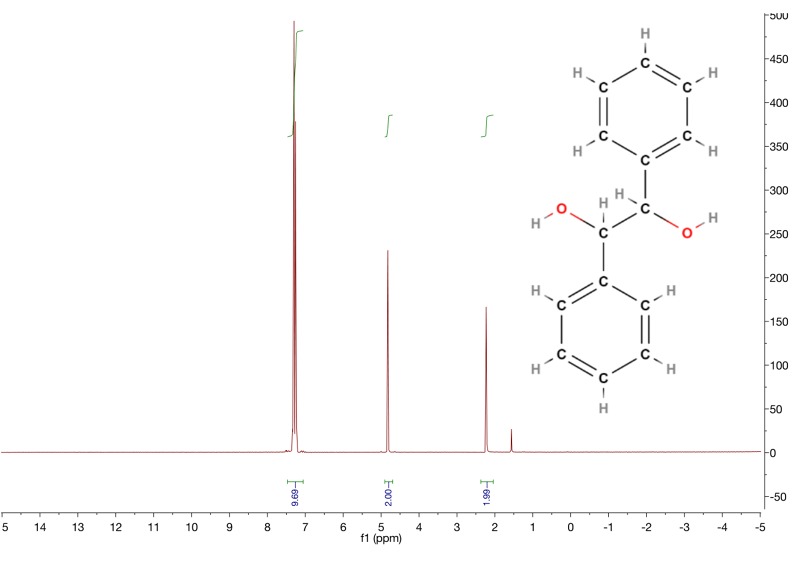
For hydrobenzoin, the non-aromatic carbons are each attached to a hydroxyl. I was wondering, which proton would be more deshielded: the one in hydroxyl or the one attached to the carbon? It seems to me that it is the hydroxyl protons, but when I looked up the ppm range of O-H, it was in 1-4 ppm range, and that didn't match up with my observations since there is another peak right around 5 that is more deshielded.