In performing ozonolysis on
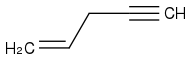
, specifically with the procedure of (step 1) O
3 @ -78 °C and (step 2) (CH
3)
2S in water, I came up with 3 different products:
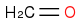
,
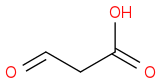
, and
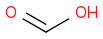
Given the subsequent reaction (reduction with sodium borohydride in methanol followed by acidic workup), I was confused as to the point of trying to generate multiple products: methanol, glycolic acid, and acetic acid. According to the book, the only product to consider is the middle one:

, with the end product (following the aforementioned reduction and subsequent treatment with hot sulfuric acid) being prop-2-enoic acid. This acid then goes on to be the dienophile in a Diels-Alder reaction.
In reflecting on this, I cannot reason as to why one would discount the methanol and acetic acid, for fear of unwanted ether and/or ester products given the hot and acidic environment once the sulfuric acid is added.
Can someone clarify for me as to why I needn't consider either formaldehyde or acetic acid in the ozonolysis of pent-1-en-4-yne? Thanks in advance.