In Stahl (DOI: 10.1021/jacs.8b05245) (I access only the abstract) I don't grasp where the hydrogen shall come from to split the N-N bond
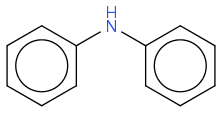

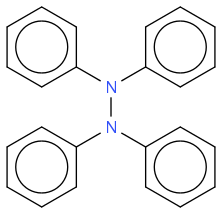
??
The 1atm O
2 supposedly serves to consume the hydrogen atoms weakly bonded to N and provoke the N-N bonding.
Once this hydrogen is bonded to oxygen, I imagine it is irreversibly unavailable.
Do I misunderstand something?
Or could it possibly be instead that the still unbonded carbazole displaces a diphenylamine from the N-N compound, gives it a hydrogen and creates the cross-coupled N-N compound? At least the curve of species concentration versus time supports this scenario.