Dear Colleagues,
The following question asks me: Calculate the Enthalpy of Reaction for ( using Hess's Law ):
C
6H
12O
6 ----->2C
2H
5OH+2CO
2Using the following data ( Enthalpy of Combustion ): Glucose=-2820 Ethanol -1367 The answer is:
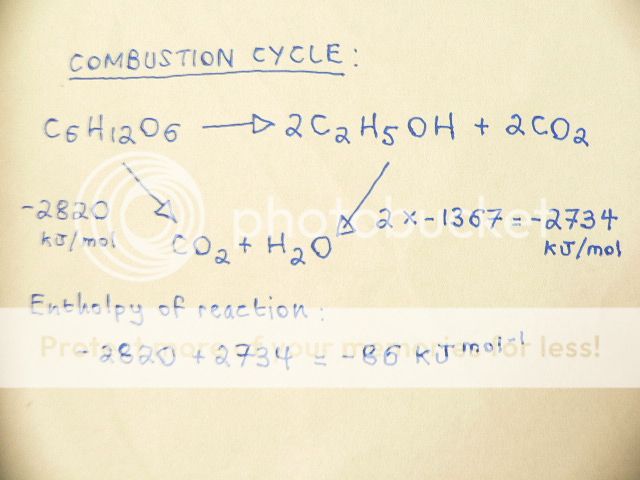
The answer then states that there is a linear method available for obtaining the same answer:
Δ
rH=ΣΔ
cH
reactant-ΣΔ
cH
reactantWhich is : -2820 - ( 2 x -1367 ) = - 86 KJ mol
My confusion is: if the question is asking for the Enthalpy of Reaction ( Delta H ) - assuming its for
the whole reaction, then why is the other product CO
2 not included in the calculation ? ( why is only 2C
2H
5OH included in the calculation ? ).