Suppose this (purely hypothetical) chemistry equilibrium:
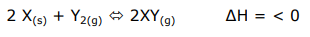
Check the following propositions:
I) the equilibrium constant becomes greater with greater temperature;
II) a greater pressure by reducing the volume increases the production of XY;
III) the addition of some X to the system increases the production of XY;
IV) the production of XY is favored by a lower temperature
Which of the propositions above are correct?
I don't know why only (IV) is correct. If I put a greater pressure, shouldn't the equilibrium shift to the side with the smallest volume, i.e., XY? And if I add X to the system, shouldn't more XY be produced?
I would have marked II, III and IV as correct, but it seems that only IV is correct.



Could you guys give me a hint?
EDIT: hey! I think I've discovered the issue! Solids do not shift the equilibrium! And the volume is only related to gases. Is this it?