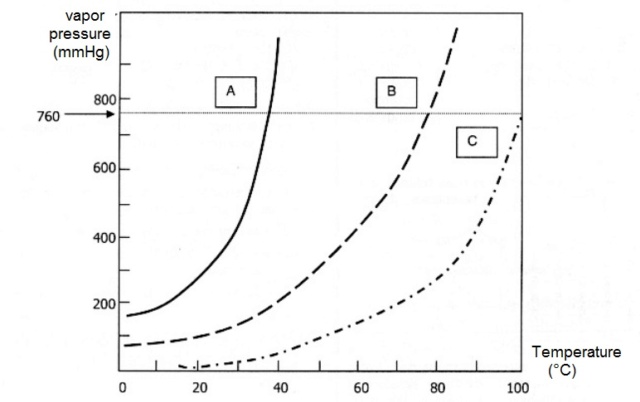
Hello
I have to tell which of these (among A, B, C) are liquids and which are gases at 80ºC e 600 mmHg. I tried converting it to 760 mmHg, because I already have that line on the graph, and that would be easier for me to understand. After all, the liquids only become gas when their vapor pressure is equal to the atmospheric pressure, i.e., 760 mm Hg.
Using Gay-Lussac's Law:
P / T = P' / T'
600/80 = 760/x
600.x = 80.760
x = 80.760 / 600 = 101.33 °C
So I think that, at 80°C and 600 mmHg (which is the same as 101.33°C and 760 mmHg), all of them--A, B, and C-- are gases.
The thing is, the answer is "only A and B are gases; C is still liquid." I don't understand. Is my book wrong?
Thank you