Hello everyone
I found this exercise that mentioned L-Metionina:
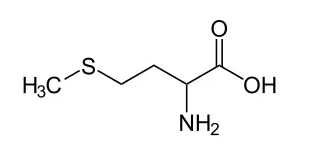
Then it proceeded to mention two propositions:
I) It has potentially nucleophilic functional groups; and
II) It has an acid-base character.
Specify which one of these is true. If both, mark both.
I think that "potentially nucleophilic functional groups" are groups that have free electrons, that is, they make covalent bonds, but they don't share all of the electrons on their valence shell. Also, they don't need electrons.
And I think that NH2 is basic and COOH is acid, so it has both characters, and I think that an aminoacid is both basic and acidic, that is, neutral.
So I think that both are correct. Am I correct?
Thank you