Hello!
My question regards the hybridization of sulfur in thionyl chloride (SOCl
2). I am also interested in finding the number of σ and π bonds in the molecule.
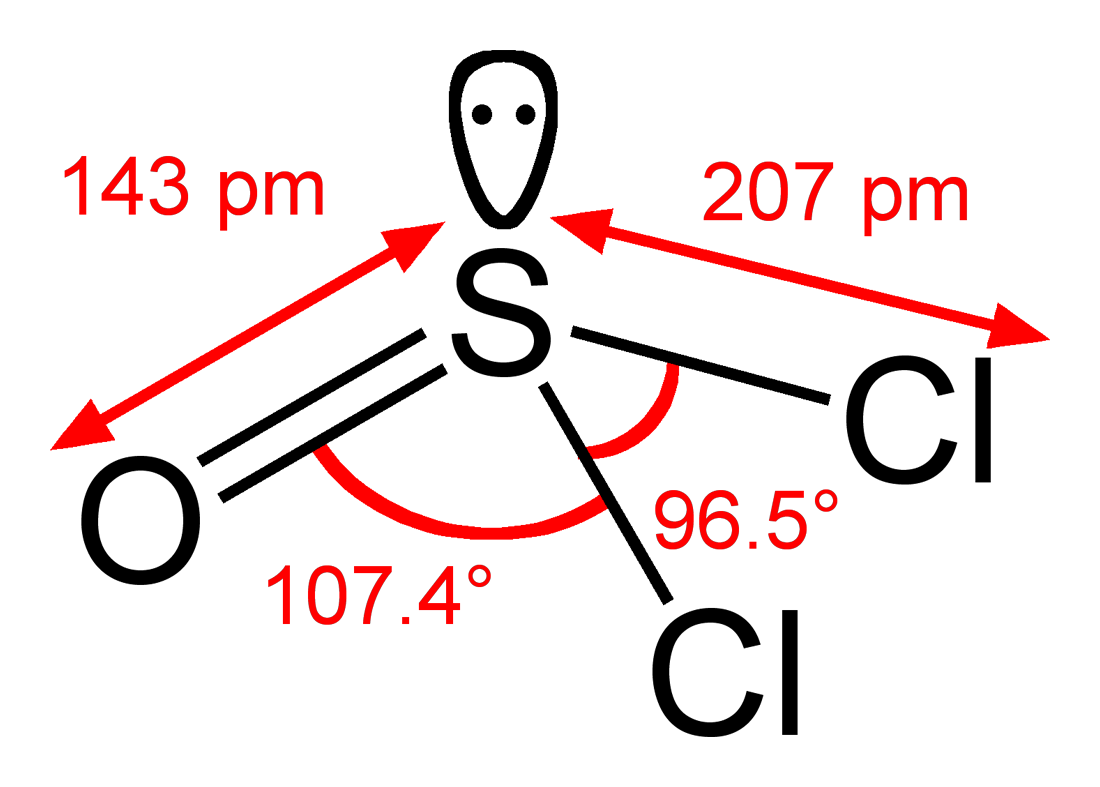
The way that our teacher has us determine hybridization is to determine the steric number of the molecule. The steric number of thionyl chloride is four. Thus the hybridization of sulfur is sp
3.
There is a double bond (1 π bond and 1 σ bond) and two single bonds (2 σ bonds). This gives a total of 3 σ bonds and one π bond.
The question is graded in 3 parts... I have two parts of three correct. You do not have to give me the answer, I was just hoping someone could guide me in the right direction.
P.S. sorry if my formatting is poor, I am new to this forum!