Hello
I've come across this exercise about the citric acid.
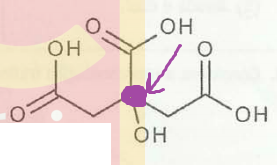
The exercise asks me to tick the correct propositions:
I - It has a good solubility in water and alkaline compounds;
II - It has only one chiral carbon;
III - It is a tricarboxylic acid.
I've painted in purple what I believe to be a chiral carbon. It seems to me that it would be so, because the OH and the C=O on both sides, right and left, of the purple dot are not "cis", they're "trans" (I think that terminology doesn't fit here, but, well, you guys understand me). So the right side is not the same as the left side.
What do you guys think about it?
The exercise also tells me (III) is false, but according to my research that's not the case. So perhaps that could be a printing error.
Could you guys please shed some light here?
Thank you