My goal is to provide a mechanism for an intramolecular Michael Addition. The reaction is as follows:
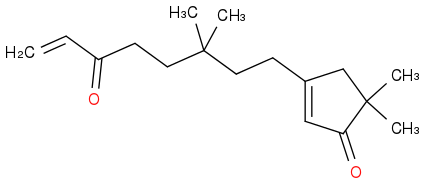
reacted with NaOET over EtOH to give
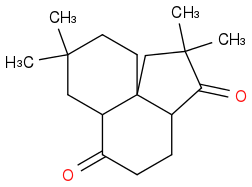
I am stumped, as I do not know whether I deprotonate the alpha carbon to the right of the first carbonyl group, or if I add the ethyl group to it. Once there, I still do not know how I am supposed to create multiple rings. Any and all help will be vastly appreciated.