1. For a single electron electrode reaction to produce one mole of substance you need to run 1 mole of electrons through the electrode - that means 96500 coulombs (so called Faraday's constant), or 96500 seconds of 1 ampere current (google Faraday's laws of electrolysis). Compare that with the output of your power supply.
2. As long as the voltage applied is too low no reactions take place, what happens later depends on the voltage applied. It can be predicted using standard reduction potentials and the Nernst equation.
3. In your setup I would expect copper to be dissolved on anode and deposited back on the cathode, so no net change in the amount of copper present in the solution. To dissolve copper you need to reduce something else on the cathode, won't be easy (for the reasons signaled in 2.)
Thanks, I think I understand what is going on. Your reply seems to prove my null hypothesis which is my original experiment dissolved the copper by some other means. I asked my teacher about the mechanics and he gave me this hint:
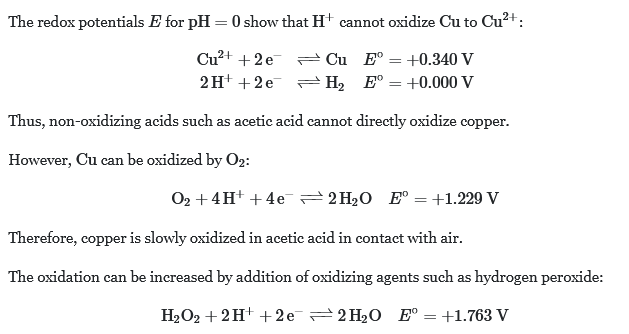
I put this together and came up with a new experiment:
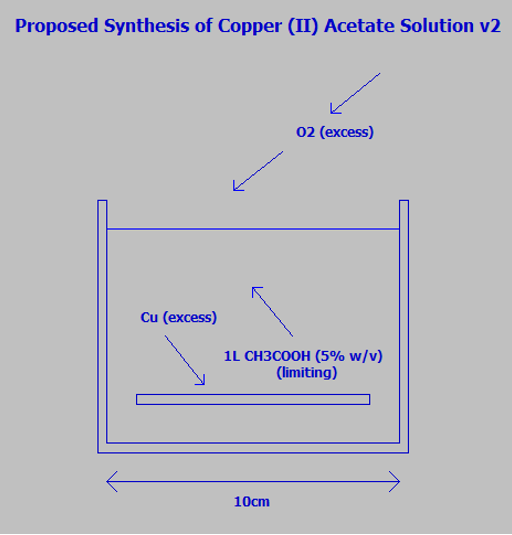
a) Balanced reaction:
2Cu + 4CH3COOH + O2

2Cu2+ + 4CH3COO- + 2H2O
b) Number of ions in starting solution:
1L CH3COOH (5% w/v - 0.83 M) contains 0.83 mol CH3COO- and 0.83 mol H+
c) Maximum mass of copper to be dissolved with excess oxygen:
? g Cu = 0.83 mol CH3COOH x (2 mol Cu / 4 mol CH3COOH) (64.5 g Cu / 1 mol Cu) = 26.8 g Cu
d) Concentration of copper ions in solution upon completion of reaction with no evaporation:
? M Cu2+ = 0.83 mol CH3COO- x (2 mol Cu2+ / 4 mol CH3COO-) (1 / 1 L) = 0.42 M Cu2+
Questions:
1) Are the previous calculations correct?
2) If the previous calculations are correct, how do I determine how much time it will take to run to completion? If I start with 1L of vinegar and excess copper, I'm assuming the rate limiting factor is the diffusion of oxygen into solution?
3) What else can we say about the experiment? For example, since I'm using nominal 5% vinegar in an uncontrolled environment, what are the greatest sources of error that can be easily controlled for?