A conformation is just the result of relative group rotation at a given temperature, within the same molecule. With configurations, you're referring to where one group is relative to another, but one isomer is not the same compound as the other.
Both share the similarity of having relative positions of groups, but the difference is that configurations are obtained by restricted rotations, forming two completely different compounds. They are not readily interconvertible. For example, CH
3CH=CHCH
3. This molecule can exist as cis and trans isomers. The cis isomer is a different compound than the trans isomer. To get from one to the other, the molecule has to have sufficient energy to break the pi bond and rotate.
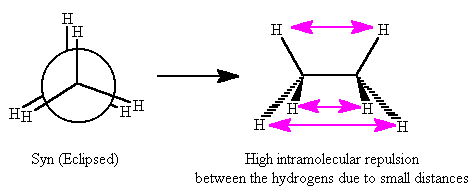
Now consider CH
3CH
3. Both the conformations shown below are different spatial alignments of the hydrogens, but both of the conformations are still ethane: