Unfortunately, I don't have the exact procedure that every group followed, but they all should have simply dissolved KAl(SO
4)
2·12H
2O in water.
I do, however, have the pH of all the solutions before the precipitate was formed.
Group 1: "The pH was found to be 4.5."
Group 2: "After dissolved the alum into water the pH was found to be 4.6."
Group 3: "The dissolved alum resulted in a pH drop of 2.7 from neutral water."
Group 4: "Based on the following equations:
Al
3+ + 6H
2O

Al(H
2O)
63+SO
42- + H
2O
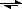
HSO
4- + OH
-A equilibrium is created and results in the following hydrolyzed reaction:
Al(H
2O)
63+ + 3OH
- 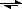
Al(OH)
3(H
2O)
3 + 3H
3O
+ Thus the solution is predictad to be acidic.
Before addition of alum: 7.0
After addition of alum: 4.3 "