This is the synthesis of an alkyne from a vinyl halide.
Compare the formulas: C8H7Cl  C8H6
C8H6
What has changed - has something been added/eliminated/substituted?
What kind of a reagent is NaNH2 (acid/base/nucleophile/electrophile)?
The Cl has been eliminated and and extra bond formed to make a triple bond.
NaNH
2 is basic isn't it? I think I can just ignore the Na? Which would make the compound NH
2-. Which would make it a nucleophile!
So would I push the electrons in the Cl-C bond onto Cl, and attack the resulting positive charge on the C atom with the nucleophile? But then that just makes an amine. I assume at this point the H
3O
+ comes into it.
So at the moment I have
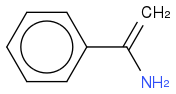
I don't think that's right. Have I gone wrong somewhere? That compound looks stable.
If I'm correct, I think that the H
3O
+ would still react with the NH
2?