Cyanogen reacts with morpholine to form a single product A.

+
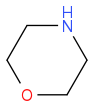

A
The spectroscopic data for A are shown below:
Carbon-13 data/ppm : 45.6, 66.2, 110.6, 142.1
IR data over 1500cm
-1/cm
-1 : 3290, 2234, 1620
Now I'm asking for help on this as I sometimes have trouble working out structures from the data in general. My attempt for this one is that I think the IR at 3290 must be either a N-H bond and so it should remain in the product. 2234 falls within the triple bond region so my guess is C#N but then I get stuck for 1620, this could either be a C=N bond or a C=O bond, my guess is that it's a C=N bond as carbonyls are usually around 1715. The problem is that I have no idea about how the reaction proceeds, I have worked out the HOMO and LUMO for both reagents but I don't know which way around they react.
Help would be much appreciated!
Thanks