Hey, everyone! I'm stuck on this problem.
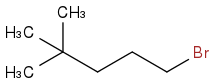
to
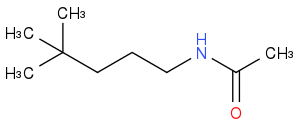
I've tried using KCN and then hydrolysis to turn it into a carboxylic acid but I then encounter a problem. I'm clueless on how to replace the carboxylic group with amide and keep the R group attached to the nitrogen. We are suppose to choose a reagent for that from a list but there is no amine, only ammonia, ammonium, and
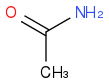
.
Not to mention after the second step that is partial hydrolysis, there is supposed to be a chain shortening according to the full problem. Any ideas?