10.1021/ol027518n
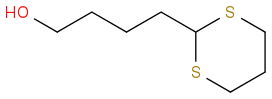

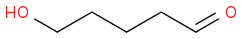
Paper says that it treated the thioacetal with 2 eq and 8 eq of DMP in 9:1 MeCN:DCM anhydrous, then aq workup afforded the product with no observable oxidation of the hydroxyl. 82% yield.
10.1055/s-1995-4114
But other groups observed this in DCM with 1.3 eq DMP; 55% yield:
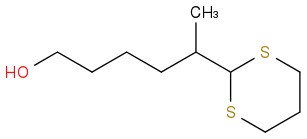

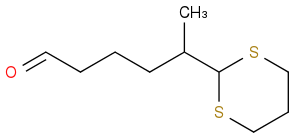
Is this just sterics? Is MeCN quenching DMP to a compound with similar valence to bis(TFA)iodobenzene?