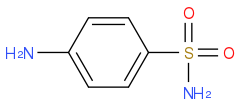
Let's look at the following sulfonamide:
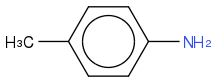
p-toluidine:
and imidazole:
![c1cnc[nH]1](https://www.chemicalforums.com/SMILES/98775fa283cf614bf396.png)
From what I learned, whenever an atom can have double bond in a resonance structure, that same atom in the overall hybrid will be best described as sp2.
In the above cases, I would say that BOTH N atoms in the sulfonamide would be best described as sp2 because of the possible resonance structures. I would also say that the N atom in p-toluidine is best described as sp2, and that both N atoms in the imidazole are described as sp2.
Is this correct? My problem is that for these structures, the resonance contributors that give double bonds to the atoms are minor. In p-toluidine, resonance contributor with sp2 broke its resonance. So how can they simply be described as sp2? I would expect more sp3 character than sp2 character in the hybrids.
In class, we covered an example similar to p-toluidine, except instead of the methyl substituent, there was an amide group (4-aminobenzamide). The N on the amino group was said to be sp2. Although the resonance form is more major since you can delocalize the electrons all the way to O in the amide group, it still requires the aromaticity to break.
So why is this true? Would it be correct to say that if there is a resonance contributor that makes an atom sp2 hybridized, no matter how minor of a contributor it is, the atom on the hybrid would still be best described as sp2?