Problem. I am supposed to show how this transformation can be done (multiple steps may be needed).
 My thoughts.
My thoughts. The brute-force method I can come up with would be to protect the ketone as an acetal (maybe with ROH to avoid transesterfication) and then reduce the ester. LiAlH
4 would give an alcohol:
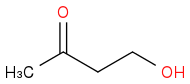
instead of the desired ether. But that can, I suppose, be solved by deprotonating the alcohol (NaH maybe?) and react it with EtBr. Deprotection with aqueous acid then gives the target molecule. However, this doesn't feel like a very neat solution. (I have thought about using something like Wolff-Kishner reduction, but that is supposed to be selective for aldehydes and ketones as far as I have learned.) Are there anything more elegant that I overlook? Maybe the β-carbonyl can be used somehow?