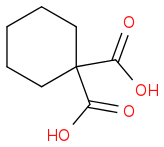
What happens if we heat it..??
I thought H
2O will eliminate ....and anhydride will form but then i realised the anyhydride which is forming , have 4 membered ring so it can't be a major product.
So now what i think this product should be major product
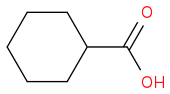
am i right ...??
What i think that anhydride formation occurs preferably in acidic medium ..otherwise CO
2 get eliminated...am i right..?
I have some doubts
1. I want to show my mechanism but i don't know how to show . anyway i am trying to
write . first C-C bond will undergo homoletic cleavage then O-H bond and CO
2 will form , Now we have H· , it can combine with C· and the product will be

. Is this mechanism is appropriate ..?.
2. Can H· attack on C-H bond to form H
2 and
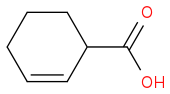
...is this our major product...??
3. What will happen if we just heat a compound having O-H bond ..This bond will undergo which cleavage either homoletic or heteroletic ...? (No acidic medium or basic medium is given)