Hi All,
I am confused about radical halogenation of alkenes and arenes with an allylic position. If I have an alkene like this:
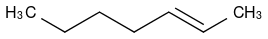
And I treat it with NBS, my textbook tells me that the bromine group will substitute a hydrogen at the allylic position, giving me this:
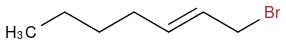
However, the same text book tells me that if I add bromine with a peroxide to a alkene like this one:
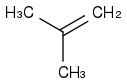
I will end up with this:
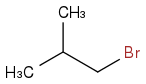
I understand both of these mechanisms, but I am confused by the competition between them in more complex molecules.
For example, in an alkene like this:
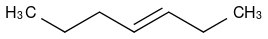
There are two potential allylic positions, neither are terminal. If I add molecular bromine without a peroxide, I will get an addition of two bromines across the double bond and an elimination of the double bond. However, from my textbook it seems that if I add HBr with peroxides I will get this:
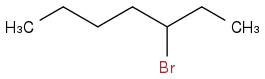
But if I do the same reaction with NBS, I will get this:
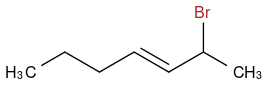
And if I do the same reaction again with molecular bromine in the presence of heat and light I will get this:
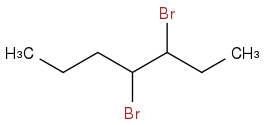
I am very confused by this. To me the three mechanisms are similar, with the one major difference that HBr has a hydrogen that can help reduce the pi bond, but a very similar question arises with molecular bromine that has been homolytically cleaved by heat and light. Is there some inherent difference between the bromine radicals from HBr, Br
2, and NBS? Is it possible to predict when a radical addition will happen across the pi bond, and when a substitution will occur at the allylic position?
Thanks!