¡Bienvenido, chocolattes! And no worry with your English.
[...] whether or not the electrons of benzene that are moving generate any radiation or electric current? [...]
If the electrons did move, they would indeed radiate an electromagnetic field, for instance light. That was a major problem at the beginning of the 20th century, as physicists modelled atoms by comparison with suns and planets. That the atoms didn't radiate, and the electrons didn't fall on the nucleus, prompted the development of quantum mechanics (QM).
The answer of QM is that electrons don't move (simplifying just some details) when they occupy some "orbitals" in atoms or molecules. Orbitals are called "stationary" for that reason. But the electrons occupy some volume because they are waves rather than points (again simplifying! Everyone agrees with "waves", opinions diverge about "are" - don't go to conflict with your professors about that). Some electrons, let's say the valence electrons (they're slightly more differentiated), take the biggest room, which is the space occupied by the atom or molecule.
In (non excited) benzene too, all electrons are stationary. Each of the six electrons drawn as three doubleD bonds plus resonance and so on sits all the time over the six carbons. So the most accurate symbol is rather
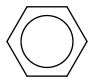
which keeps the complete symmetry. In fact, drawing any bond between only atom pairs is already an abuse - but it's much more comfortable.
Electrons are not always stationary. Such situations are solutions of Schrödinger's equation for electrons too. For an electron trapped in an atom, a molecule, a region of a semiconductor component... we know that such non-stationary solutions can be written as weighed sums of stationary solutions. And because in such situations, with movement, the electron radiates hences loses energy, they're transitory. After attoseconds to minutes, the formerly excited electron has joined a stationary situation (an "orbital" in the case of an atom or molecule) where it doesn't radiate. This explains why stationary solutions are especially interesting.
QM makes very few assumptions, and uses the same kind of equation for electrons in atoms, in a semiconductor, alone in vacuum... and for other particles. With that, it gives explanations like "why doesn't benzene radiate" and makes numerical predictions that are accurate. As disadvantages, it is sometimes non-intuitive, and its solutions result from computer software except for the simplest situations, but this theory is a Rolls-Royce that represents the world as we observe it, so it is the basis of present physics and chemistry.