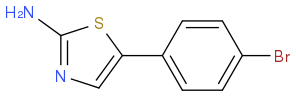
I've been trying desperately to synthesise the above thiazole via this intermediate:
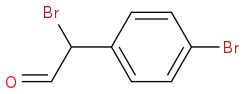
However, I do not seem to be able to produce the corresponding aldehyde as I think it is condensing with itself. I have tried a Dess Martin, SO3.Py/DMSO/Et3N and a Swern.
On another note, Reaxys gives me these conditions, but I do not think the product is drawn correctly i.e. I think the reaction would yield a 2,4 substitution pattern.
