As far as I could dig up, Aqua Regia is an admixture of Nitric Acid (NHO3) with Hydrochloric Acid (HCl), usually at the ratio of 1:3, but sometimes at 1:4.
Nitric Acid is a potent oxidizer, but can dissolve only an imperceptible amount of Au
(s) via oxidation of Au
(s) into its ion Au
3+. Then hydrochloric acid will reacto to gold forming [AuCl4]
-, that will remain as a ion in the aquaous phase instead of precipitating to the bottle. The reaction is:
Au
s + 3 HNO3 + 4 HCl
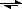
[AuCl4]
− + 3 [NO2] + [H3O]
+ + 2 H2O
I wasnt taught yet about the requil reactor, but a quick search pointed it to indicate a reaction which will fastly reach equilibrium point. Ill certainly research this deeper later on when I get back home.
I cant say why uric acid wont dissolve gold, but from what I have found so far, gold wont dissolve in other acids due the geometry of its crystal structure when in solid state, which are too stable to be provoked by other ions. But its interesting to note how gold and silver will alloy naturally, but again, I have to research this deeper on why this happens.
Uric acid seems to be a cyclic amine that went under esterification reaction, thus the =O appended to the cyclic carbon chain. Ive found an enzimatic pathway for the metabolization of purine into uric acid in humans, but I havent had the time to read it so far as we were only assigned this task yesterday and I had other stuff to do.
Something that isnt clear for me: how to determine which compounds would have priorit in reaction? More electronegative compounds would react first with less electronegative ones? This sounds a lame question, but there's alot I cant get off the top of my head back from high school times. Also, there's the thing on polar vs non-polar molecules, of which it seems uric acid would have characteristics similar to ethanol regarding these aspects. Is that accurate, or am I missing something from this?
Thanks for the attention!

EDIT: the reaction is supposed to be ideal, with 99.99% gold