We might need more information
like how are you combining these
Are you heating this
Are you cooling the reaction
Are you adding one to the other drop wise
are you using any solvents
are you doing this in open container or have air in the system initially
I assume you are trying to do this reaction
NH
2SO
3H + NaNO
2 
NaHSO
4 + H
2O + N
2or
H
3NSO
3 + NaNO
2 
NaHSO
4 + H
2O + N
2Can we also assume you understand the properties of the reactants
For instance
Sulfamic acid
https://en.wikipedia.org/wiki/Sulfamic_acidIUPAC name Sulfamic acid
CAS Number 5329-14-6
Chemical formula H
3NSO
3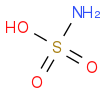
Molar mass 97.10 g/mol
Sulfamic acid melts at 205C (401F; 478K)
before decomposing at higher temperatures to
H
2O, SO
3, SO
2, and N
2.
Appearance white crystals
Solubility in water Moderate, with slow hydrolysis
Sodium nitrite
https://en.wikipedia.org/wiki/Sodium_nitriteCAS Number 7632-00-0
Chemical formula NaNO
2![N(=O)[O-].[Na+]](https://www.chemicalforums.com/SMILES/bda44d41711cdc9af89f.png)
Molar mass 68.9953 g/mol
Melting point 271C (520F; 544K)
Above 330C sodium nitrite decomposes (in air) to
Na
2O, NO, NO
2Appearance white or slightly yellowish solid
Solubility in water
71.4 g/100 mL (0C)
84.8 g/100 mL (25C)
160 g/100 mL (100C)