I am a fan of your pathway with sulfonyl isocyanates. If the amine is the unreactive one, then it better have a really hot partner.
Would making the sulfonyl chloride in order to get to the sulfonyl isocyanate (via potassium cyanate) be too difficult?
If you want to stick with the carbamate route, perhaps some of the transamidation procedures in the literature would help. Unless you have a strong enough base to deprotonate the amine, I dont think a stronger base is going to get you there. Its a matter of the electrophile/nucleophile pair in my mind. Carbamate is a crappy electrophile, so you need a powerful nucleophile, or some unique conditions, to get it to convert to the urea. I would also worry in general with this pathway that the amine would transamidate the wrong way and give you the urea of the pyrimidine instead of the coupling reaction, since the sulfonamide is probably a better leaving group than ammonia.
Very helpful, thank you. I can make the sulfonyl chloride easily (I make the sulfonamide through the chloride in one pot starting with the corresponding thiol), so your suggestion for making the isocyanate should be doable. Looking back at my lab book, I had been using a few different procedures, one which I am fairly confident worked as far as the isocyanate, as I could see it by LRMS after work up of what should have been the sulfonylurea (
https://pubs.acs.org/doi/full/10.1021/jm301501k?src=recsys). I couldn't convince my aminopyrimidine to go through to the sulfonylurea using their conditions (all I got back was starting material), but I also didn't have time to play around with them more.
I had considered using NaH with the carbamate pathway to try and deprotonate the amine, although I am not convinced it is a good option. Your transamidation suggestion is something I hadn't considered, so I may look at that also if I can't get the isocyanate to work for whatever reason.
It looks like some pharmaceuticals are made with this connections (I assume thats your research area). For the synthesis of Tolazamide it looks like they just heated the amine with the sulfonyl carbamate neat (with excess? I didnt do the math).
https://en.wikipedia.org/wiki/Tolazamide https://patents.google.com/patent/US3063903
"C. N '4-CHLOROBENZENESULFONYL)-N -HEXA- METHYLENEIMINOUREA FREE BASE 1 A mixture of 11.41 g. of 1-aminohexamethyleneimine and 26.0 g. of 4-chlorobenzenesulfonylurethane (Marshall et al., supra) was heated at 130 C. (oil-bath temperature) for 2 hours. The resulting ethanol and unreacted amine were removed at 100 mm. pressure for 1 hour and at 20 mm. for 2 hours While keeping the oil bath at 130 C. The residue was cooled and recrystallized from methyl ethyl ketone, giving 20.53 g. (66%) of N-(4-chl0robenzenesulfonyl)-N'-hexamethyleneiminourea free base in the form of colorless prisms melting at 196-199 C."
Yeah, there is a good amount of literature on molecules even closer in structure to mine that Tolazamide. The issue seems to be that the particular 2-aminopyrimidine I am using is especially non-nucleophilic (even for pyrimidines, which are already not very reactive) and just does not like to react in the ways others describe. Reacting the carbamate (or isocyanate) neat with the amine and heating the crap out of it in a pressure vessel is something I have scrawled in my notebook from last year when I was doing this as a point for further investigation. Thanks for bringing the patent to my attention.
I've had some issues in the past reacting substituted 2-aminopyridines with some electrophiles. For example, in trying to form a 2,3-diketopiperizine by reacting the following with oxalyl chloride:
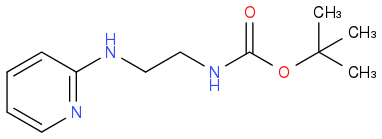
The reaction turned BRIGHT blue, but on work-up only the starting material was isolated. Eventually we worked out we were forming the following pyridinium species in situ, and it was promptly hydrolyzing on silica/work-up/the second we looked at it. I presume this happens because the sp2 pyridine nitrogen was actually the most nucleophilic, and the by-product was stable enough under the reaction conditions not to further react to the product through a DMAP-like pathway.
![O=C1[N+]2=C(N(C1=O)CCNC(OC(C)(C)C)=O)C=CC=C2.[Cl-]](https://www.chemicalforums.com/SMILES/62c33e7ec49ba64c4815.png)
In the end we had to redesign our route, so unfortunately I can't offer you any insightful words of wisdom.
In my experience, these pyrimidines are (thankfully) not overly prone to reaction on the ring nitrogens. The amine nitrogen *should* be the most reactive in the molecule, but it is something to watch out for. You have given me something to think about for another reaction that was giving me some odd results though, so thank you for that.