I hadn't found quickly a mixture of water and antifreeze that melts at -80°C. Not propylene glycol, not ethylene glycol, not erythritol, not glycerol, but possibly a
mixture of several glycols in water, remembering that ethylene glycol is a bit toxic. Nasa's attempts were possibly in this direction, rather than an alkane, if they sought compatibility with polyethylene.
Besides water and alcohols,
alkanes offer a big fusion heat, fluoroalkanes less so. They are not always compatible with polyethyleneS and polypropyleneS, but with other usual plastics they are. Non-flammable criterium suggests the molecule shall be bigger than 2,6-dimethyloctane, and melting point criterium tells it must be branched.
Farnesane is a first idea
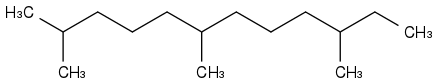
bp=+243°C mp<-71°C fp+109°C wow, so it does burn with a wick or as a mist, but matches won't light a leak on concrete. Other sources tell mp around -100°C. Farnesane makes 99,76% of the AMD-200 fuel attempt by Amyris, where many kg were produced. My feeling is that the company meanwhile targeted easier markets than jet fuels.
BEWARE the syntheses I suggest in the linked threads are NOT tried and I'm no chemist, so they can be complete cr*p.
2,4,6-trimethyl-dodecane has phase-change properties similar to farnesane. It seems rare too.
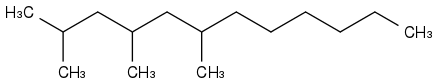
Unless a company like Amyris decide to produce these compounds, they are expensive lab rarities, so synthesising would be cheaper. And if you produce such an alkane, other uses and customers await you.
JP-10 is mass-produced
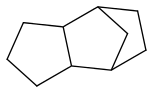
it melts at -79°C from NIST (Chickos et al is doubtful), and its flash point is very little above +55°C. Its kerosene smell can be misleading.
Grafting an alkane tail to cheap myrcene, then saturating everything, could be a flexible design, as the tail's length adjusts the melting point. Here myrcene
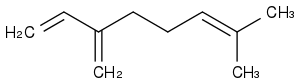
Do not believe melting points in general. Measures are rare, most values are software estimates, but these fail grossly on melting points.