Hello, everyone!
There is this exercise about the production of acetylene.
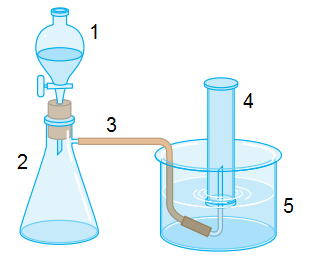
If we add CaC
2 and H
2O together, we will have acetylene and Ca(OH)
2. Ok, I know this. But the question is about the laboratory equipment.
My book says: fill with water (4) and (5); put water on (1); put CaC
2 on (2).
I don't understand the first step. Why should we fill with water (4) and (5) before everything? When water droplets leave (1) and fall on CaC
2, it makes acetylene. It is a gas, and it can't go back to (1), because water is dripping. So it goes though the hose up to (4). Where is my mistake?
Thanks